Raising awareness is vital as it helps individuals recognize if they are experiencing a condition they can potentially treat. Unfortunately, many rare diseases lack cures or therapies due to a significant shortage of participants available for clinical research. The uncommon nature of these diseases poses considerable challenges for patients, their families, and healthcare providers trying to achieve an accurate diagnosis and provide optimal care.
What constitutes a rare disease?
A rare disease refers to any condition that impacts a small portion of the population. In certain regions, an orphan disease is a rare condition that is so uncommon that it receives little to no funding or research focus, lacking financial motivation from government entities or other organizations. Orphan drugs are treatments specifically designed for orphan diseases.
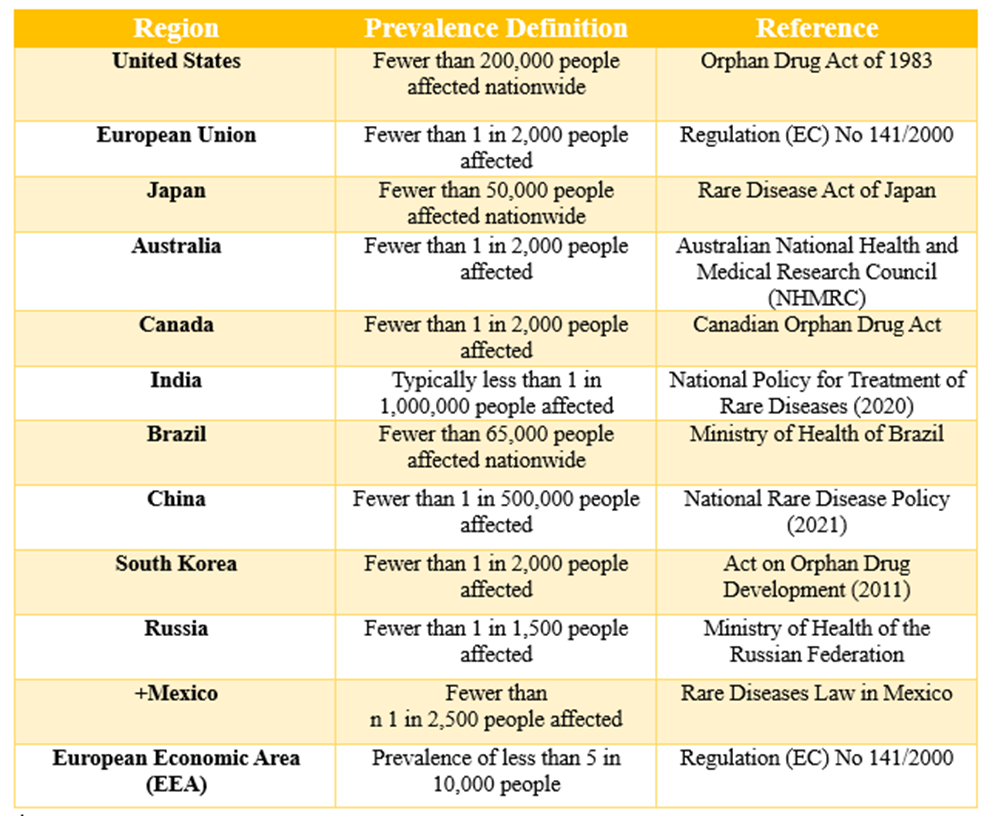
There are approximately 6,000 to 8,000 rare diseases, with 250 to 280 new conditions documented each year, affecting an estimated 6 to 8 percent of the global population. A rare disease is defined as a condition that afflicts fewer than 200,000 individuals in the United States. Collectively, over 10,000 rare diseases impact more than 30 million Americans. There is no single, universally accepted definition for rare diseases. Some definitions focus solely on the number of individuals affected by a disease, while others factor in aspects such as the availability of effective treatments or the severity of the condition.
Prevalence of rare diseases across the world
Globally, rare diseases afflict 3.5% to 5.9% of the population. This suggests that 263 million to 446 million people worldwide suffer from a rare disease. According to RARECARE’s European population-based rare cancer registry statistics, 4,300,000 persons in the European Union have a rare cancer diagnosis. This represents 24% of all cancer diagnoses in the EU.

Image source: https://globalgenes.org/rare-disease-facts
What are the causes of rare diseases?
Most rare diseases are genetic in nature, which means they last a person’s entire life, even if symptoms do not appear right away. The vast majority of uncommon diseases (about 80%) are inherited. This means that parents often pass it on to their children.
However, genes can change on their own. Many rare diseases appear in infancy, and around 30% of children with uncommon disorders die before the fifth birthday. Fields’ illness is the rarest known ailment, affecting only three persons, two of whom are identical twins. Ribose-5-phosphate isomerase deficiency is considered the second-rarest, with only four cases reported in 27 years.
Onset of rare disease according to age
Rare diseases exhibit a diverse range of symptoms, which may seem unrelated at first glance. Rare medical ailments can affect individuals at any stage of life; whether in childhood, adulthood, or both. A study on rare diseases conducted by Wakap et al. (2020) identified 6,172 distinct rare diseases. Among these 6,172 rare diseases, Wakap et al. (2020) discovered that:
- 69.9% (3,510 rare diseases) occur exclusively in children,
- 11.9% (600 rare diseases) manifest solely in adults,
- and 18.2% (908 rare diseases) have an onset that includes both pediatric and adult populations.
Diagnosis of rare diseases
“The average rare disease patient consults with 5 doctors and receives 3 misdiagnoses”
Many rare diseases mimic common ones and share genetic pathways, but their presentation is typically more aggressive or severe.
“On average, patients with rare diseases wait 4 years for a final diagnosis. Better understanding of these diseases may lead to better diagnostics and therapies for more common diseases.” Obtaining an accurate diagnosis is sometimes a complex and time-consuming process, as physicians and caregivers frequently lack the necessary expertise in an illness that they rarely face.
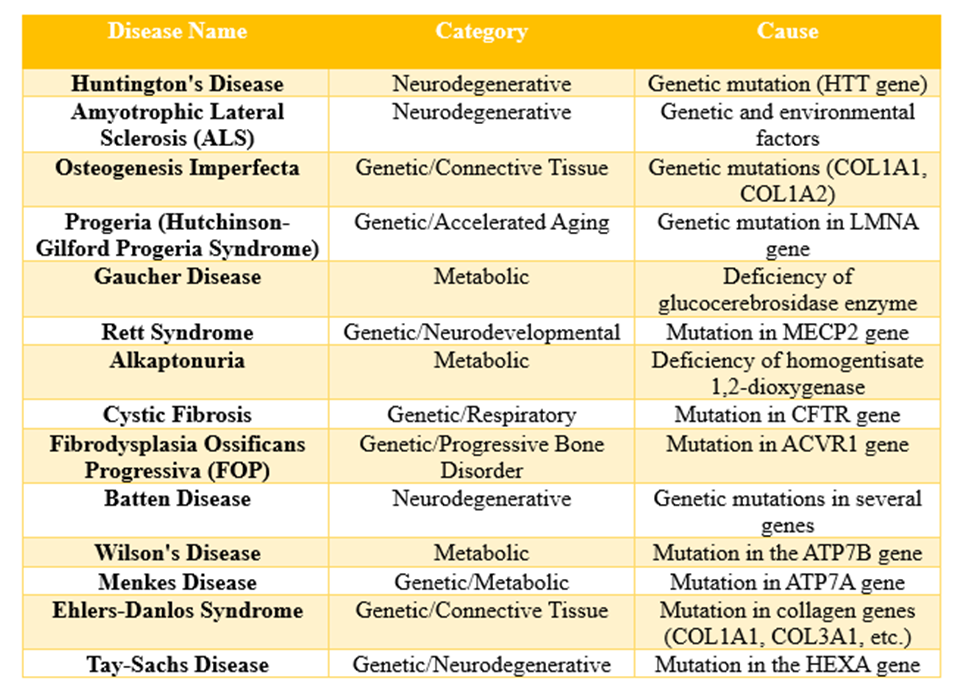
Examples of some rare diseases
How many rare diseases have treatment?
There is no consensus on the number of rare diseases that have treatments, but stakeholders estimate that about 95% lack a licensed option, leaving only 5% with available therapies. Effective treatments are often scarce and expensive due to scientific, manufacturing challenges, and small markets, creating access barriers.
What All rare diseases have common
All rare disease patients share a common challenge: limited medical understanding and research. This often leads to misdiagnosis, delayed treatment, and inadequate support, making the experience isolating for both patients and their families. Rare conditions deserve more attention from doctors and researchers.
Role of NGS in rare disease diagnosis and treatment
Most rare diseases have a genetic origin, making the identification of genetic variants essential. In the mid-2000s, linking mutations to diseases became challenging due to the need for unrelated individuals with shared variants and similar symptoms. Data sharing faced obstacles from competitive environments and consent limitations, while inconsistent phenotype terminology complicated patient matching. However, next-generation genomic techniques, like whole-genome sequencing demonstrated by Kristina Ibañez and colleagues, have shown promise in identifying rare neurological disorders tied to repetitive DNA sequences. Early genetic diagnosis is crucial for improving health outcomes and lowering treatment costs for rare diseases.
Orphan drug act and rare diseases
Before the US Orphan Drug Act of 1983, just ten orphan medications were approved throughout a decade. Over the next 25 years, 247 medicines were approved for more than 200 uncommon disorders.
In Europe, the European Commission Regulation 141/2000 led to 63 orphan medicinal products gaining authorization by 2010. However, despite the increase in orphan designations, only about 15 products were approved annually for fewer than 300 rare diseases. By 2010, around 400 orphan products were available, but the FDA noted a slowdown in innovative therapy development due to challenges such as small clinical study populations and unclear outcome measures.
What Is the NORD® Rare Disease Database?
The National Organization for Rare Disorders (NORD) focuses on the identification, treatment, and cure of rare diseases through education, advocacy, research, and services. Its Rare Disease Database, created in collaboration with MONDO, Orphanet, and OMIM, includes detailed reports on symptoms, causes, diagnosis, treatments, and support resources, with Spanish translations available. For diseases lacking a report, information from reputable sources is provided, though consulting a healthcare provider is recommended.
The International Rare Diseases Research Consortium
The International Rare Diseases Research Consortium (IRDiRC), established in 2011 through discussions between Dr. Ruxandra Draghia‐Akli and Dr. Francis Collins, aims to enhance collaboration among funders, advocacy groups, and researchers to improve rare disease diagnostics and develop new therapies. Its ambitious goals include creating 200 new therapies and improving the diagnosis of most rare genetic diseases by 2020. IRDiRC continues to address ongoing challenges in the field.
Rare disease-related mortality
According to the 2016 National Vital Statistics Report in the United States (Heron, 2018), birth defects, deformations, and chromosomal disorders contributed to 22.2% of all neonatal deaths, 20.8% of all infant fatalities (the leading cause in this group), and 10.7% from 1 to 4 years of age (all ethnic groups and races, both sexes). In Japan, congenital conditions and chromosomal disorders caused 35.7% of infant fatalities.
A report from the 2005 European Conference on Rare Diseases investigated the life expectancy of 323 rare diseases and determined that 25.7% are potentially fatal before the age of five, 36.8% have a shortened life expectancy, and just roughly a third (37.5%) live a normal life.
Challenges to Patients
Patients with rare diseases face three challenges: they may experience disease manifestations but struggle to find physicians who are knowledgeable about what they are experiencing and how to manage them; they may suffer the disease’s consequences and go completely unnoticed; and they may face extremely high costs for disease-specific medicinal products.
Refrences
Ferreira, C. R. (2019). The burden of rare diseases. American journal of medical genetics Part A, 179(6), 885-892.
Nguengang Wakap, S., Lambert, D. M., Olry, A., Rodwell, C., Gueydan, C., Lanneau, V., … & Rath, A. (2020). Estimating cumulative point prevalence of rare diseases: analysis of the Orphanet database. European journal of human genetics, 28(2), 165-173.
Haendel, M., Vasilevsky, N., Unni, D., Bologa, C., Harris, N., Rehm, H., … & Oprea, T. I. (2020). How many rare diseases are there?. Nature reviews drug discovery, 19(2), 77-78.
Dawkins, H. J., Draghia‐Akli, R., Lasko, P., Lau, L. P., Jonker, A. H., Cutillo, C. M., … & International Rare Diseases Research Consortium. (2017). Progress in rare diseases research 2010–2016: an IRDiRC perspective. Clinical and translational science, 11(1), 11.
Groft, S. C., Posada, M., & Taruscio, D. (2021). Progress, challenges and global approaches to rare diseases. Acta paediatrica, 110(10), 2711-2716.
Aymé, S., Kole, A., & Groft, S. (2008). Empowerment of patients: lessons from the rare diseases community. The lancet, 371(9629), 2048-2051.

MPhil, PhD Microbiology
Follow me ⬇️





Post Comment